VivoSense continues to lead the way in infant respiratory analysis.
Orange County, CA, February, 2017 – Washington University School of Medicine, and St Louis Children’s Hospital with funding from the NIH Prematurity and Respiratory Outcomes Project (PROP) https://clinicaltrials.gov/ct2/show/NCT01435187 have published early research findings. The primary goal of the PROP study was to identify biomarkers and clinical variables in premature infants that would potentially predict pulmonary status up to one year later.
Premature infants typically have reduced respiratory function and up to 45% of premature infants are at risk for chronic lung disease (CLD). The PROP study used Respiratory Inductance Plethysmography (RIP) to non-invasively acquire data that describes chest wall movement. VivoSense® software was used to analyze these respiratory patterns and to generate and investigate potential biomarkers and clinical indicators.
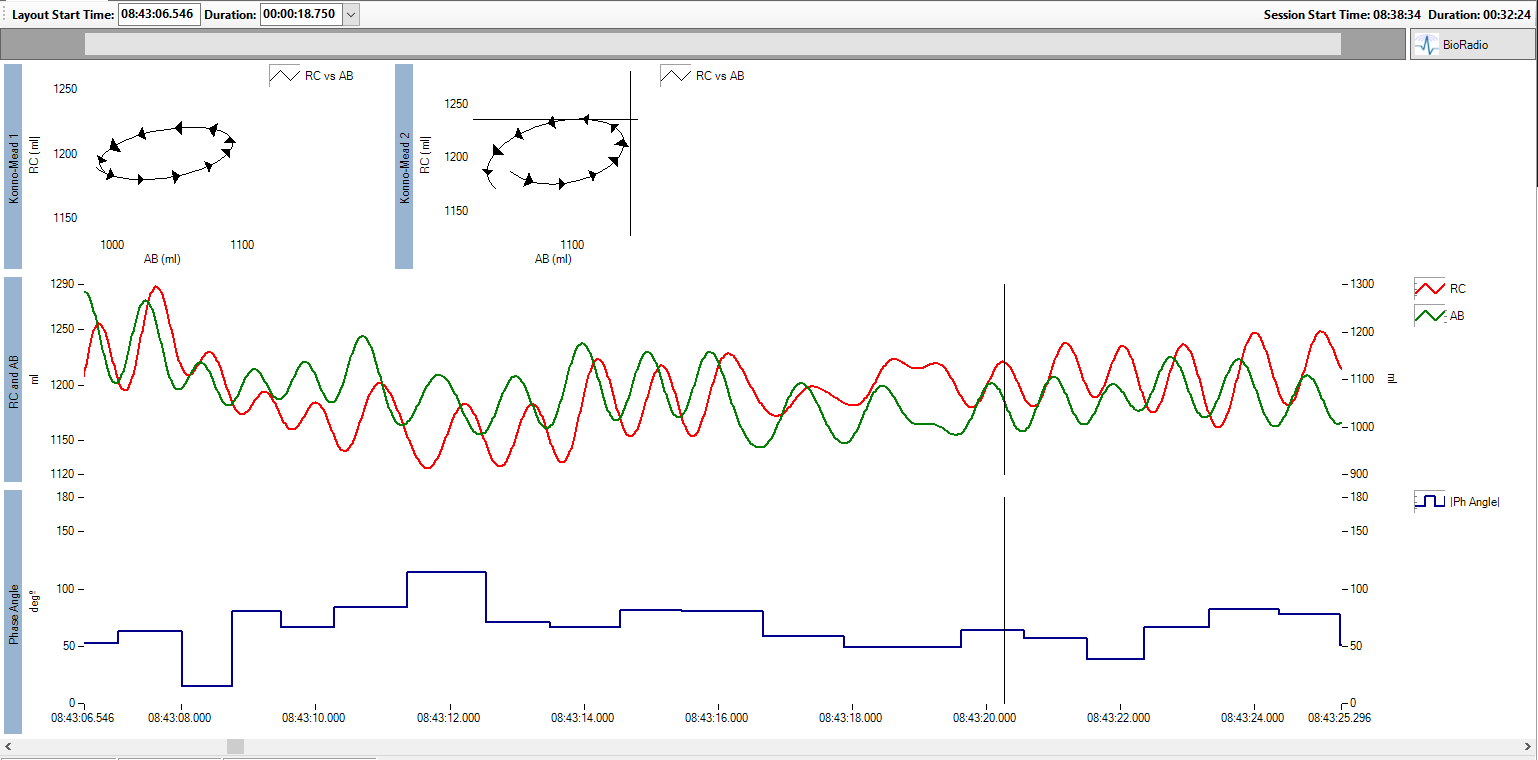
RIP signal displaying lack of synchrony between Rib Cage and Abdominal airflow
The study found that premature infants display a lack of synchrony between their rib cage and abdomen due to airflow obstruction and poor respiratory compliance. Appropriate measurement and quantification of the degree of rib cage and abdominal synchronization provides valuable knowledge regarding lung properties and infant prognosis and could help understand and guide the course of supportive interventions.
Respiratory inductance plethysmography (RIP), provides an easy to apply investigational tool that offers a non-invasive assessment of respiratory synchrony and other valuable metrics. Pulmonary analysis traditionally requires the use of devices such as masks or mouthpieces coupled to the airway opening which are unsuitable for monitoring premature infants.
“We are pleased to provide the sensor and analytic solutions that facilitate these important studies,” says Lance Myers PhD, Vivonoetics’ Chief Science Officer. “We look forward to new publications and findings from our academic research partners that will help advance understanding and treating respiratory complications in premature infants.”
Please visit our Publications page to see more publications using VivoSense®.
Dudley Tabakin
Dudley Tabakin, MSc. is Chief Product Officer and co-founder of VivoSense and a fervent believer in “good data” over “big data” in the development of digital endpoints from wearable sensor technology.

