Infants with Spinal Muscular Atrophy (SMA) develop respiratory insufficiency due to progressive respiratory muscle weakness caused by degeneration of alpha motor neurons in the spinal cord. This weakness results in ineffective airway clearance (obstruction), which is the major cause of morbidity and mortality in infants with SMA. Thus, measurement of longitudinal changes in respiratory muscle function in infants and young children with SMA-1 is critical to the evaluation of interventions targeting SMA-1.
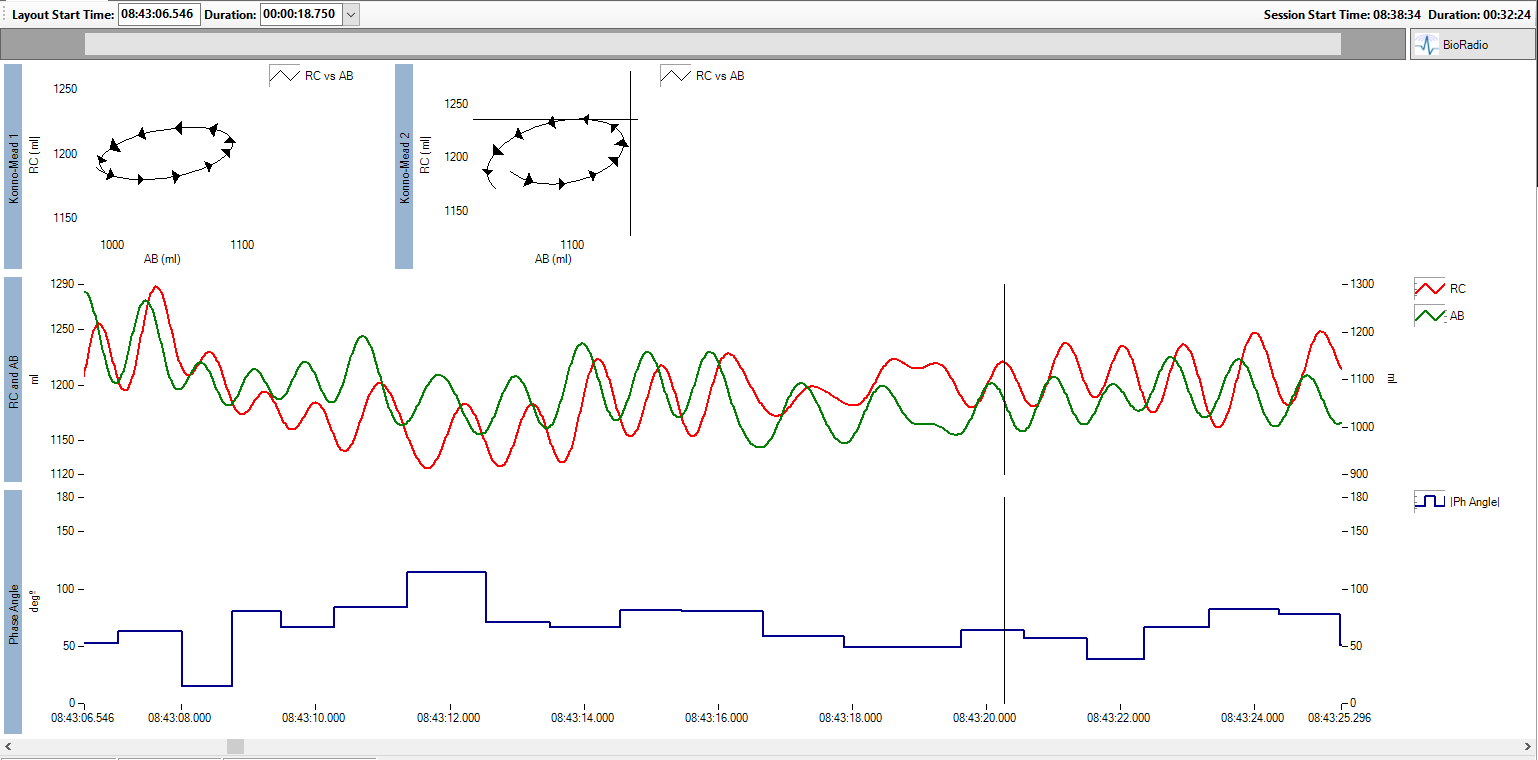
Accurate measurement of obstructive breathing can be obtained by examining the degree of asynchrony between abdominal and rib cage breathing. In this case study, VivoSense provided accurate and objective respiratory assessment services for a large multi-center clinical trial for a SMA-1 therapeutic. Infants were instrumented with Respiratory Inductance Plethysmography (RIP) sensors using the BioRadio. Appropriate breaths are selected using Kono-Mead plots (upper chart), phase relation between the breathing compartments (bottom chart), and abdominal and rib cage breathing movements (middle chart).
Dudley Tabakin
Dudley Tabakin, MSc. is Chief Executive Officer and co-founder of VivoSense and a fervent believer in “good data” over “big data” in the development of digital endpoints from wearable sensor technology.